Solved Chemical Equilibrium Le Chatelier's Principle
Chemistry 12 Unit 2 - Chemical Equilibrium Worksheet 2-2 - LeChatelier's Principle Page 2 6. Hydrogen peroxide is decomposed as follows: H2O2(l) H2(g) + O2(g) DH = +187 kJ Predict the direction of equilibrium shift by each of the following imposed changes: a) Increase the [H2].Answer _____
Le chatelier practice q and answers Le Chatelier’s Principle Practice 1) For the reaction

28 PRACTICE PROBLEM. At 358 K the esterification of an organic acid and an alcohol has a forward reaction rate constant of 2.25×10−4 M−1•s−1 and a reverse rate constant of 1.85×10−4 M−1•s−1. When the temperature is increased to 368 K, the rate constants for the same reaction have values of kf = 3.14×10−4 M−1•s−1 and.
Le Chateliers Principle Worksheet (1) CHM 1083 Studocu

Le Chatelier's Principle Practice Interactive. If a system at equilibrium is affected by certain factors, such as changes in volume, pressure, temperature, et cetera, then this principle states that the reaction that corresponds with the system will shift in such a manner to counteract that change and return to equilibrium.
SOLVED Worksheet 2 LE CHATELIER'S PRINCIPLE Le Chatelier' Principle states that when system
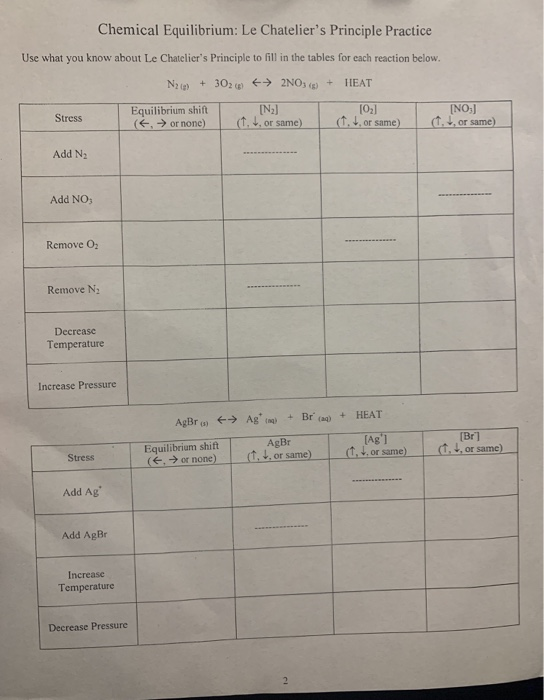
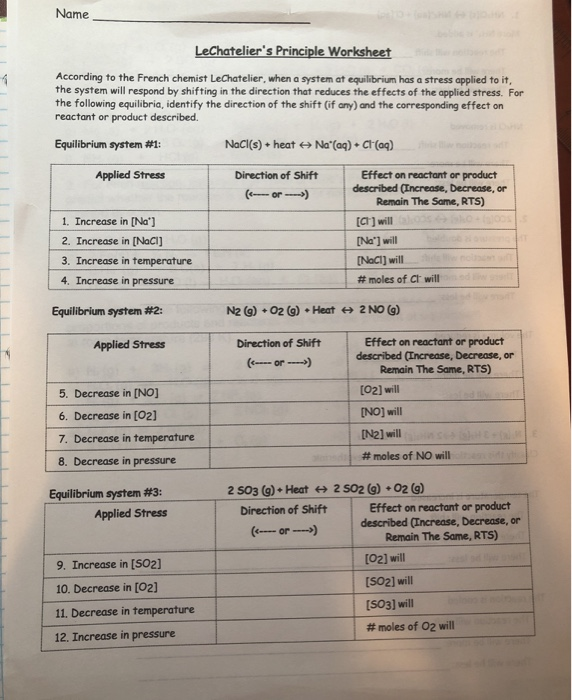
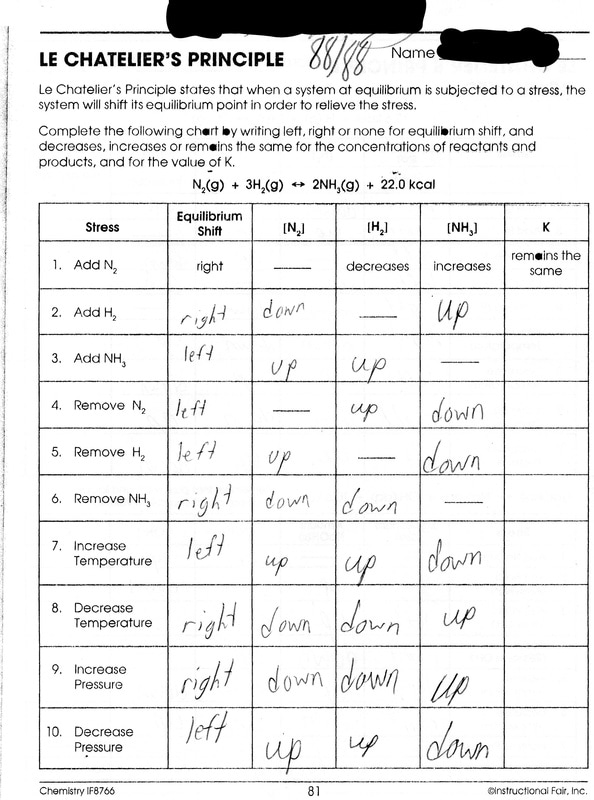
KMBT 654-20140224095350. LE CHATELIER'S PRINCIPLE Name Le Chatelier's Principle states that when a system at equilibrium is subjected to a stress, the system will shift its equilibrium point in order to relieve the stress. Complete the following chart by riting left, right or none r equilibrium shift, and ecreases, Increases or remains the same.
Le Chatelier'S Principle Worksheet Answer Key 2020 2021 Rocco Worksheet
Le Chatelier's Principle Worksheets 2024. One of the fundamental ideas of chemical equilibria, which describes what happens to a system when something quickly removes it from a state of equilibrium, was put out by the French chemist and engineer Henry-Louis Le Chatelier in 1884. It is known as the Le Chatelier's Principle, which is actually.
Free Printable Le Chatelier's Principle Worksheets

Le Chatelier's Principle Worksheet. 1)1) For the reaction below, which change would cause the equilibrium to shift to the right? Decrease the concentration of dihydrogen sulfide. Increase the pressure on the system. Increase the temperature of the system. Increase the concentration of carbon disulfide. Decrease the concentration of methane. 2.
Le Chatelier's Principle Continued Worksheet
Using Le Chatelier's principle. The following reaction is allowed to reach equilibrium. What happens to the reaction when some CaCO 3 ( s) is removed? Loading. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of.
Free Le Chatelier's Principle Worksheets for Students

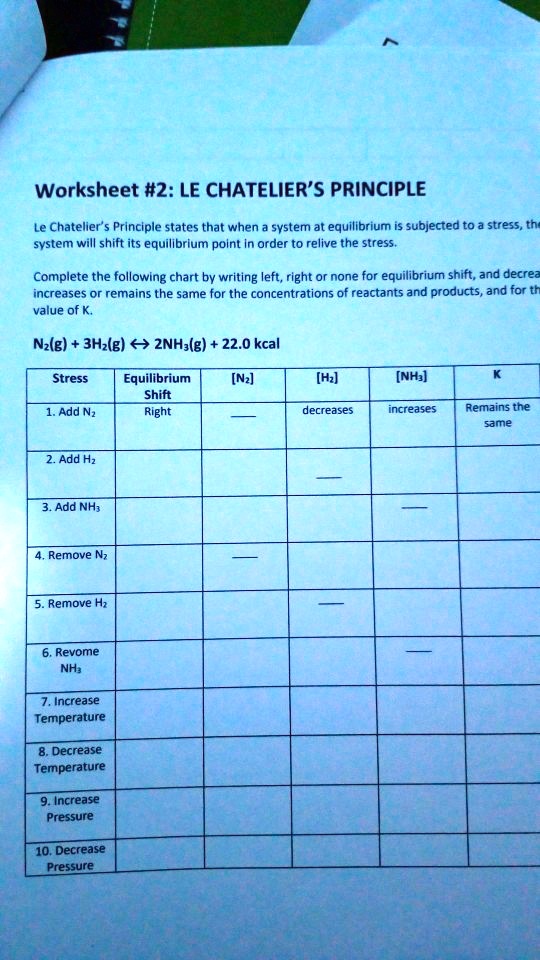
Le Chatelier's Principle Worksheet KEY 1. Balance the equilibrium reaction shown below and predict whether the equilibrium will shift towards products or reactants under the changing conditions below. 2 NaHCO3 (s) + heat ↔ Na2CO3 (s) + CO2 (g) + H2O (g) the temperature is lowered: Reactants reaction is endothermic in forward direction, lower T favors reactants.
Le Chatelier's Principle Practice Worksheet Answers

Quiz & Worksheet Goals. These tools will assess your knowledge of: The premise of Le Chatelier's Principle. Increase in the concentration of the reactants. Exothermic reaction. Pressure on a.
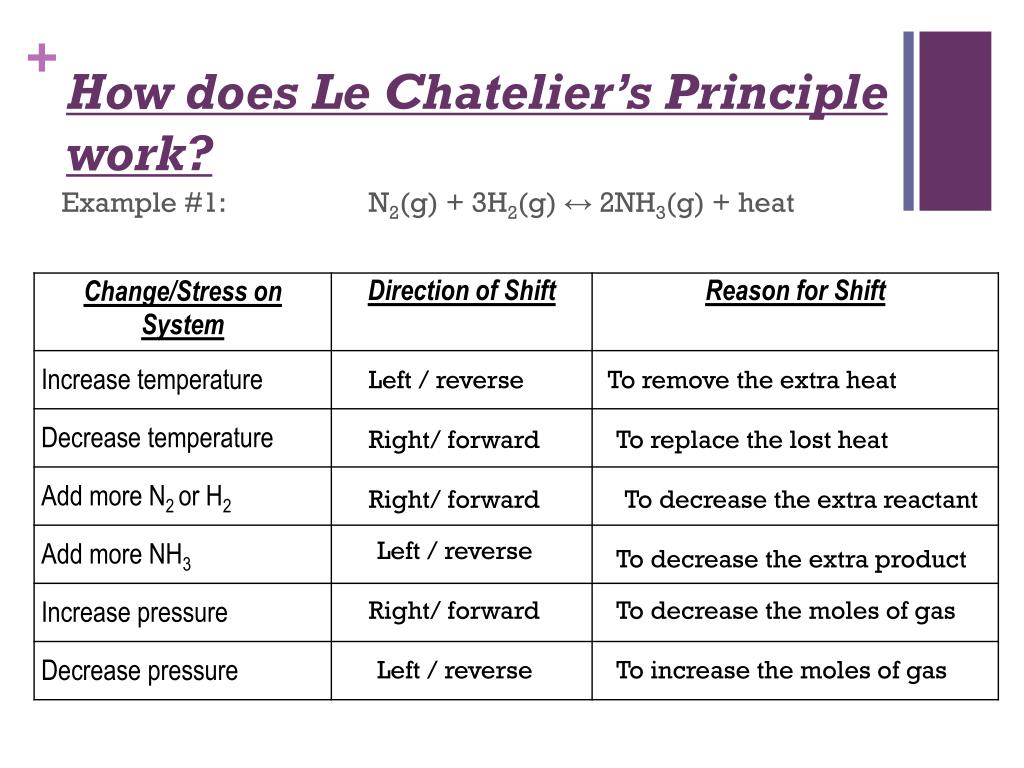
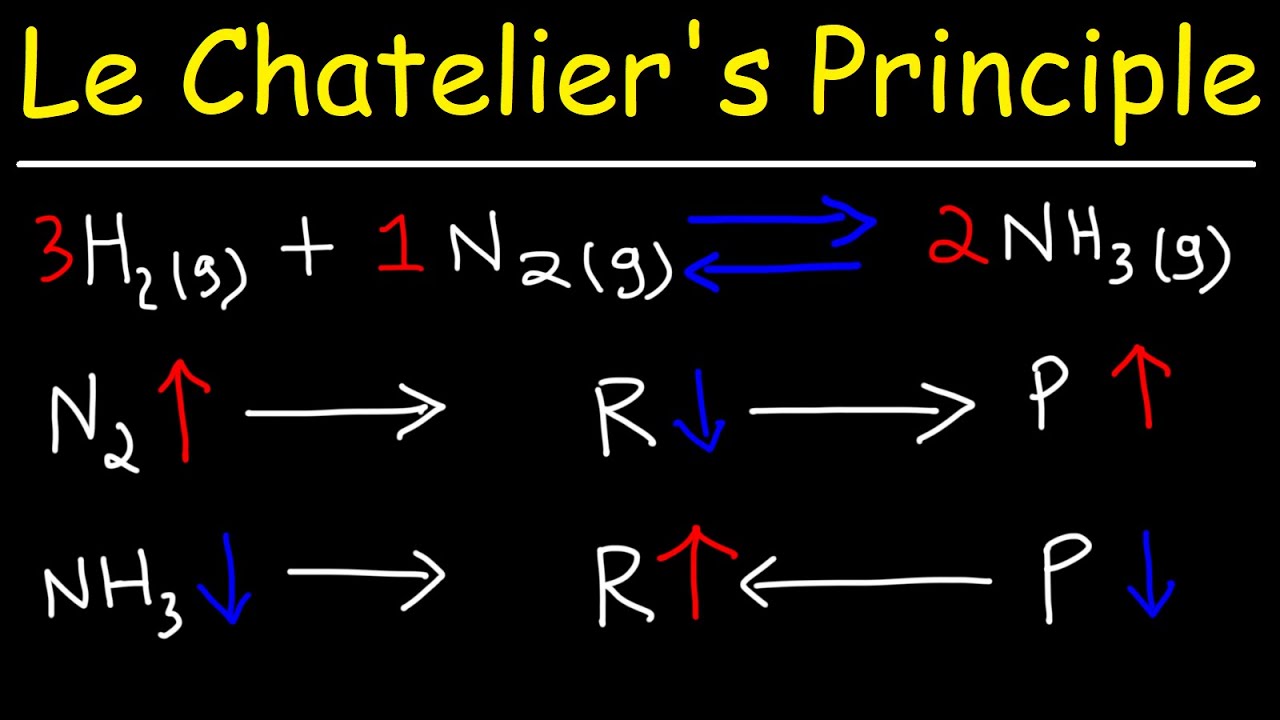
Le Chatelier's Principle YouTube
Lab Worksheet for "Chemical Equilibrium and Le Chatelier's Principle". General Instructions: Complete Part A, Part B Steps 1a-1e (skip 1f) and Steps 2a-2e (skip 2f-2i). Follow the procedure in the lab manual and record your data on this worksheet. As your laboratory report, turn in to your TA this worksheet along with the appropriate pages from.
le chatelier's principle Chemistry Practice Worksheet CHEM 1060 U of G Studocu

Practice le principle name chem worksheet stress that is applied to reaction that is at equilibrium conditions will shift the equilibrium position in direction
Equilibrium and Le Chatelier’s Principle Worksheet and Le Chatelier’s Principle Worksheet As you

Worksheet for completion to consolidate of equilibrium constants calculations. chemguide questions le principle state le principle. ethanoic acid and ethanol
Free Printable Le Chatelier's Principle Worksheets

opyriht 21 eoria ublic roadcastin. ll rihts reserved. se or distribution by an unintended recipient is prohibited. Name ate nit 1 ractice roblems II e hateliers rinciple
Le Chatelier's Principle Worksheet 2 Answers
4. Consider the following equilibrium process for the commercial production of hydrogen: CO ( g) + H 2 O ( g) ⇆ CO 2 ( g) + H 2 ( g ) Δ H ° = +42 kJ/mol. Predict the direction of the shift in equilibrium when. (a) the temperature is raised. (b) more CO gas is added to the reaction mixture.
Chemistry Page 2
Worksheet: Le Chatelier's Principle Name_____ CHEMISTRY: A Study of Matter © 2004, GPB 12.11 If a system at equilibrium is subjected to a _____ , the equilibrium is
Free Le Chatelier's Principle Worksheets for Students

LE CHATELIER'S PRINCIPLE CONTINUED Name 2Hl(g) 12.6 kcal Equilibrium Shift right 2. 3. 4. 5. 8. 9. 10. Stress Add H2 Add 12 Add HI Remove 1-12